A novel bioactive-fibrinogen wound healing dressing for craniofacial injuries
Battlefield wounds present a unique challenge due to extended evacuation times and unique infection that often complicate the healing process. Currently traditional bandages are used on the battlefield to dress wounds. They provide a physical barrier and help to control hemorrhage but do not actively aid in the healing process. Navy researchers are working to create bandages with bioactive components such as growth factors, antibiotics and other agents that may enhance healing rates and reduce scar formation. Improved wound healing rates resulting from these bioactive dressings could lead to fewer dressing changes resulting in better patient outcomes such as reduced trauma and fewer complications.
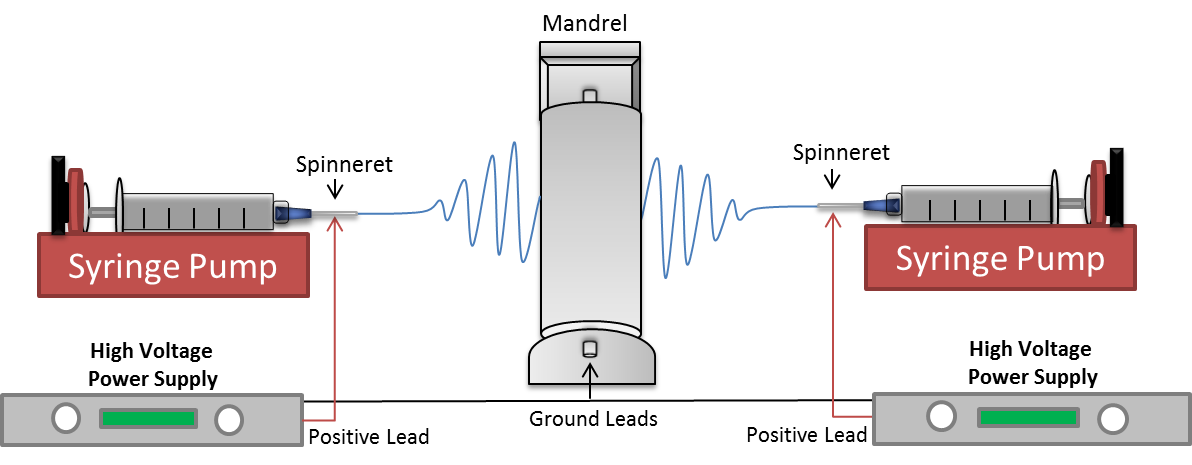
Navy engineers and scientists at NAMRU-SA have constructed an electrospinning device that can be used to make dressings created from polymer nanofibers. In this process, biologically compatible natural and synthetic polymers are dissolved with bioactive agents into a solvent, and then spun into a wound dressing using electrical forces. The resulting wound dressing slowly biodegrades releasing the bioactive compounds over time. The released molecules can be tested to determine if they are able to improve or even accelerate the healing process. The development of a bioactive antibacterial scaffolds to significantly improve wound healing outcomes and the patients’s quality of life would be critical in providing a platform for a new generation of wound dressings.
The purpose of this study is to develop, fabricate, and evaluate the chemical and physical characteristics of chitosan (CS) /poly ethylene oxide) (PEO) /fibrinogen nanofibrous scaffolds for wound dressing applications. Additionally, the loading and release of platelet-derived growth factor (PDGF) from the scaffolds will be explored. The goal is to determine if the release of PDGF will enhance wound healing and, potentially reduce scar formation.
Navy researchers’ results showed that a non-woven CS/PEO/FI composite nanofibrous scaffolds can be fabricated with fiber diameters of approximately 200 nanometers. The addition of CS/PEO to fibrinogen significantly strengthens the composite allowing it to be handled with ease. Additionally, the data showed successful loading and release of PDGF. Due to fibrinogen’s natural ability to bind PDGF, two different release profiles were observed from the individual components. Finally, the data showed that the scaffolds were non-toxic to mammalian fibroblasts and reduced both bacterial attachment and growth.
Combined CS/PEO/FI composite nanofibrous scaffolds were successfully developed and fabricated using the electrospinning technique depicted below. Additionally, PDGF can be successfully incorporated into the scaffold. Findings from this study will be used to test the efficacy of released PDGF for wound-healing applications. The ability to electrospin nonwoven antibacterial CS and FI-based nanofibers designed for controlled drug-delivery would be an important step in the development of a stable antimicrobial natural polymer-based wound dressing for clinical use.